Clinical
- Sudden onset of fever (pyrexia ≥38°C), or recent history of fever, and cough or sore throat.
- Other symptoms can include rhinorrhoea, limb or joint pain, headache, vomiting or diarrhoea.
At risk groups
- Chronic respiratory, heart, kidney, liver, neurological disease
- Immunosuppression
- Diabetes mellitus
- People aged 65 years and older
- Children <2 years
- People on medication for asthma
- Severely obese people (BMI≥40)
- Pregnant women (including up to 2 weeks post‐partum)
- Haemoglobinopathies
- Children with any condition (e.g. cognitive dysfunction, spinal cord injury, seizure disorder or other NM disorder) that can compromise respiratory function, especially those attending special schools/day centres
Admission indicators
- Respiratory distress
- Severe dehydration or shock
- Altered level or consciousness or other neurological symptoms
- Significant co‐morbidity
- Rapidly progressive or unusually prolonged illness
- CXR findings
- Immunocompromised
- Social issues
Investigations
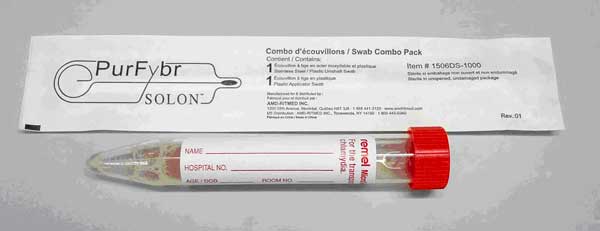
"Clinicians at MUH are now (January 2015) able to test for Influenza in the microbiology laboratory, Mercy University Hospital. This test will be performed using an appropriately labelled nasal swab (Red capped “Remel” swab, see photo). Clinical details must be provided.
Testing will take place in duplicate during the validation process (the second swab will be sent to the National Virus Reference Laboratory Dublin for confirmation). During this period please send 2 clearly labelled swabs where influenza is suspected- one to be a nasal swab and one to be a combined nasal and throat swab.
This test will be available during the core laboratory service 8am to 8pm Monday to Friday and weekends 9am to 12pm.
Please inform the laboratory if testing for other respiratory viruses is clinically indicated."
Dr Deirdre O’Brien, Consultant Microbiologist, MUH
Management
- Institute Infection Control Precautions
- Involve ID team
Needs admission
- Antiviral treatment is recommended for hospitalised patients
- Take nose and throat viral swabs
- Chemoprophylaxis for close contacts is not generally recommended. Chemoprophylaxis may be considered appropriate in some residential settings, such as nursing homes, special education residential centres (discuss with local public health).
For discharge
- Consider antiviral treatment if patient is in a defined risk group (above) or if patient is particularly ill.
- Treatment should be started as early as possible (preferably within 48 hours of onset) but may be started at any time if clinically indicated.
Infection control precautions
Hand Hygiene
- Alcohol rub/gel and or soap and water if hands are physically dirty.
- Place a surgical mask on the patient if tolerated.
HCWs PPE
- ≥ 5 years: Surgical mask
- < 5 years: Surgical mask, gloves and apron/gown
- Aerosol generating procedure (AGP) : FFP2 or FFP3 mask, gown, gloves and eye protection.
Patient Placement
- Assessment in single room if available.
- If no single room, maintain at least 1metre separation from other patients where feasible.
- Avoid nebulisation in communal areas
- Prioritise use of a single room for assessment of patients with ILI.
Chemoprophylaxis
- Chemoprophylaxis for close contacts is not generally recommended.
- Chemoprophylaxis may be considered appropriate in some residential settings, such as nursing homes, special education residential centres (discuss with local public health).